Welcome, 2024!
As we step into the fresh start of a new year, it’s time to take a moment to look back at some of the milestones that shaped our 2023.
Expanding Capabilities
- Launch of LARC Robotics Venture
The year kicked off with the launch of LARC Robotics, our venture into the field of Endourology. The project leverages our extensive expertise in Interventional Radiology, aiming to simplify renal access in the early treatment of kidney stones. LARC Robotics means advancing beyond a surgical tool by developing a comprehensive digital surgery system to address common challenges in PCNL procedures.

Interventional Systems’ CEO, Pedro Costa, further explained:
“Our robot simplifies gaining renal access as it allows for targeting under real-time fluoroscopy, and it will be offered in combination with a digital platform that will allow surgeons to visualize patient-specific, high-fidelity 3D reconstructions of the diagnostic scans to prepare for the procedure. The final component is a data and analytics module for post-procedural analysis, to find areas of opportunity and performance trends.”
This groundbreaking initiative highlights our commitment to innovation, contributing to a paradigm shift towards unparalleled precision and efficacy in endourological interventions.
- Micromate™: 510(k) Clearance for CT Optical Navigation
Later in the year, we secured 510k clearance for Micromate™ for percutaneous procedures using CT Optical Navigation. This landmark approval positions Micromate™ as the first needle-based intervention robot in the U.S. market compatible with both fluoroscopy and computed tomography. Our miniature robot was also cleared for integration into third-party navigation stations.
- ATEC Spine Acquires Interventional Systems’ Miniature Robotic Platform
Back in April, Alphatec acquired the REMI assets, which include our miniature robot, for spine fusion procedures. Interventional Systems remains the exclusive manufacturer and R&D provider for the robotic platform in the spine surgery field.
Partnering with an established strategic company like Alphatec further validates the need for our technology in the market. We are proud to join efforts to integrate our technology into ATEC’s portfolio and look forward to contributing with our expertise in image-guided procedures.
“We are specialists in robotics, that’s what we do. We believe that all companies should be open to partnership, because that will allow them to access new fields that they wouldn’t explore otherwise. It offers possibilities, minimises risks and exposes you to different markets. That allows you to differentiate yourselves. We owe that to our shareholders, and ultimately to the patients. That’s why we decided to do this.” – Pedro Costa, in The Surgibots Podcast.
You can listen to the full episode here.
Accelerating Market Adoption
- LARC Robotics: First PCNL Cases
The first PCNL surgeries were performed at the Landeskrankenhaus Hall Hospital, in Austria, shortly after LARC Robotics launch was announced. Since then, our experts have been working towards not only validating technical and clinical claims in both Europe and the US, but also further developing the technology to address other challenges in the workflow.
“Everything went as expected with the surgeries that we already performed. It was “plug-and-play”, and the benefits of robotic-assisted targeting were evident. We’re working with one of the most seasoned and high-functioning teams I’ve ever had the chance to witness, and it is a pleasure and a source of great learnings to continue developing the platform”, stated Pedro Costa.
At the end of the year, we were honored to attend the Prerecorded Surgery Session featuring our miniature robot, led by Prima. Prof. Dr. Udo Nagele at the Technology and Training in Endourology Course. While in Torino, we collected valuable feedback that helped us better understand how our technology can further improve the outcomes of percutaneous nephrolithotomy procedures.
- Micromate™ arrives in India!
Still in October, our team had the pleasure of installing the Micromate™ system and providing initial training for future users in India, together with our partner Meril Life. Our Clinical Product Specialists supported multiple cases in four different hospitals and demonstrated how our robotic needle guidance and navigation system empowers clinical teams to be more efficient and deliver the best results every time.
- CFDA Clinical Trials Initiated in China
In China, two clinical trials kicked off during the year for CFDA approval:
- HicRen started conducting clinical trials in spine fusion with leading Hospitals in the field of Spinal Surgery.
- Minimax Medical Holding Group took the lead in conducting a clinical trial for CT-guided IR procedures in the Greater China region.
The mission is to provide clinicians with an unparalleled robot-assisted surgical solution that offers accuracy, efficiency, and cost-effectiveness. This endeavor not only aims to develop state-of-the-art medical technology but also to make it more accessible to a broader segment of the population, underscoring our commitment to pioneer better patient outcomes through Value-based Robotics.
Clinical Outcomes
After our first CT-guided cases with Dr. Marco van Strijen in the Netherlands, which proved a seamless transition of the workflow to the CT room, several other hospitals have adopted the Micromate™ robotic system for their CT-guided procedures. Our Clinical Product Specialist team focused on working alongside these clinical sites across Europe to collect data on a total of 61 procedures assisted by our planning and navigation station.
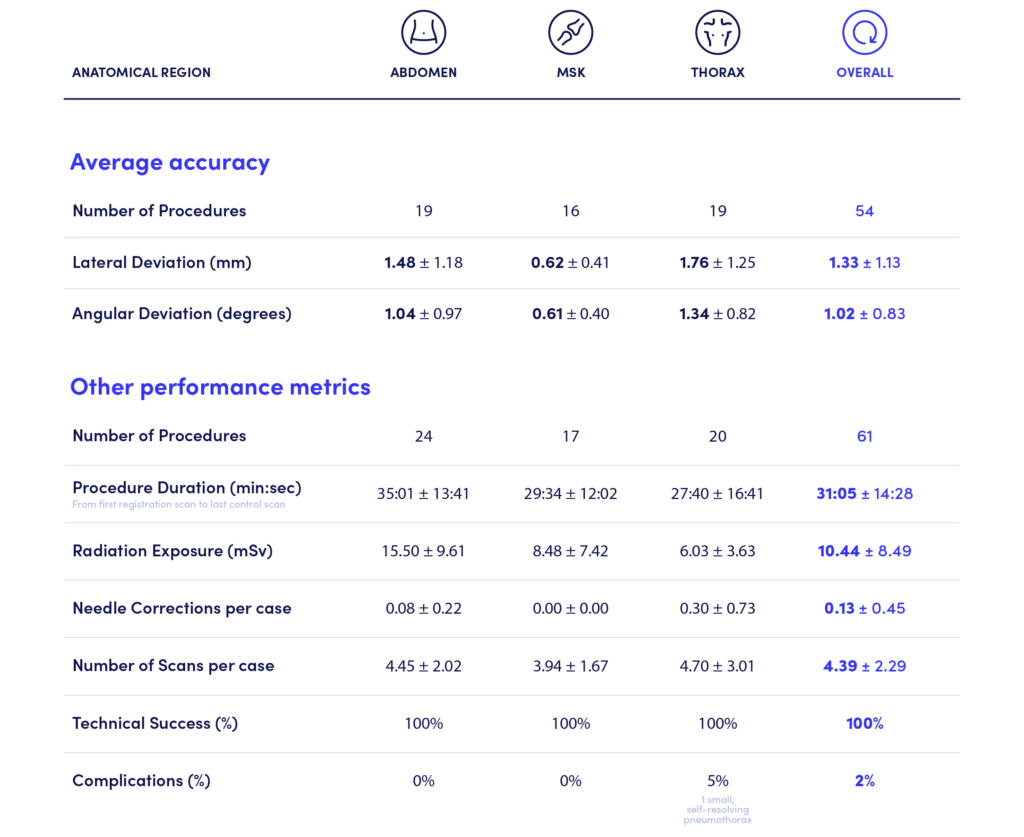
In the reported cases, Micromate™ supported 1.33 ± 1.13 mm average lateral deviation and 1.02 ± 0.83º average angular deviation to the planned trajectory, validating the consistency and accuracy of the robotic platform.
The report compiles data from three anatomical regions with 100% technical success, 2% complications, and an average radiation exposure of 10.44 ± 8.49 mSv.
Showcasing Micromate™
Throughout the year, we had the chance to attend several Congresses and participate in valuable courses, which allowed us to connect with some of the brightest minds in our industry and engage in insightful conversations.
ECIO and CIRSE stand out as key events for enabling our team to establish new connections and bring together the physicians of our Centers of Excellence in person.
Moreover, our attendance at AUA in Chicago marked LARC’s official debut in an Industry Congress, adding a significant milestone to our journey.
Looking into 2024
We ended 2022 on the following note: “Here’s to a 2023 filled with innovation, collaboration, and accomplishment toward a future of better patient outcomes through micro-invasive interventions!”
Our team’s dedication has turned this aspiration into reality, exceeding expectations in a year marked by significant change and growth for Interventional Systems.
Embracing the belief that there’s never too much of a good thing… Here’s to another year filled with innovation, collaboration, and accomplishment toward a future of better patient outcomes through micro-invasive interventions!
A huge thank you to our team, partners, and customers for making this year a remarkable one.